There are several significant concerns for acquiring a strong validation system for speedy microbiological methods (RMM):Sterility testing, By itself, are not able to function evidence of complete sterility with the solution. Nonetheless, it does serve as a crucial validation action in the bigger sterilization and excellent Command protocols.Direct
About duct work for hvac
We know this duct also requires a quantity circulation rate of 0.79m3/s so we will utilize the velocity and quantity circulation price to discover the missing knowledge.We’ll include things like a full worked illustration in addition to applying CFD simulations to optimise the effectiveness and effectiveness making use of SimScale. Scroll to the
Detailed Notes on process validation in pharmaceuticals
By carefully monitoring the process, potential difficulties can be resolved proactively, reducing the risk of solution non-conformities and making sure dependable merchandise excellent.Modify Regulate is a life span monitoring strategy. Scheduling for perfectly executed alter Handle strategies includes the subsequent elements:“Process validation
How prescription of medicines can Save You Time, Stress, and Money.
Typically moments, the precise power you need is not available, so the pharmacist will substitute an suitable option in your case.The https:// makes certain that you will be connecting into the Formal Internet site and that any information and facts you supply is encrypted and transmitted securely.chief elements on the prescription. Also in the ins
FBD usages in pharmaceuticals Can Be Fun For Anyone
C. Fluidization- Growth-Retarding Chamber: Enlargement and Finger Bag chamber may be the exact same, which facilitates Fluidization, along with, arrests the powder from flowing out through the finger bag filters. The fluidization chamber provides a inspection window Or possibly a look at glass. The underside in the chamber and the very best of
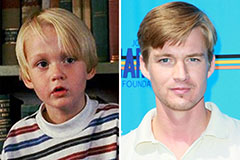 Mason Gamble Then & Now!
Mason Gamble Then & Now! Andrew Keegan Then & Now!
Andrew Keegan Then & Now! Jurnee Smollett Then & Now!
Jurnee Smollett Then & Now! Michael Jordan Then & Now!
Michael Jordan Then & Now! Loni Anderson Then & Now!
Loni Anderson Then & Now!